Repository
The SimInSitu consortium is sharing some outcomes of the development activates with the interested community to facilitate the further development of cardiovascular in-silico models. Therefore two deliverables, from work-package 4 and 7, were designated as Open Research Data Pilot and are made publicly available: a Patient-Specific Benchmark TAVI in-silico platform (SimInSitu D4.10) and an in-silico Virtual Clinical Trial platform (SimInSitu D7.4).
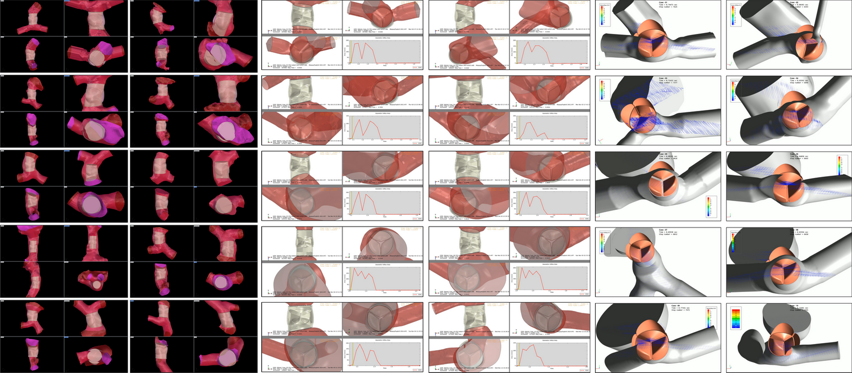
Patient-Specific TAVI In-Silico Platform
Patient-specific modelling of the treatment of a stenotic aortic valve by transcatheter aortic valve implantation (TAVI). The credibility of the proposed patient-specific TAVI model is established through model development aligned with ASME V&V40 guidelines. The patient-specific TAVI model encompasses the deployment of the Sapien 3 (S3) Ultra device, followed by a post-TAVI analysis of bioprosthetic hemodynamics. This public repository is built with the intent to disseminate the in-silico methodology to assess the S3 device structural and haemodynamic performance. The user can run different patient-specific cases to assess the impact of patient boundary conditions (i.e., blood pressure), material properties of the aortic root, and device implantation depth.
The repository is accessible via the following web-address:
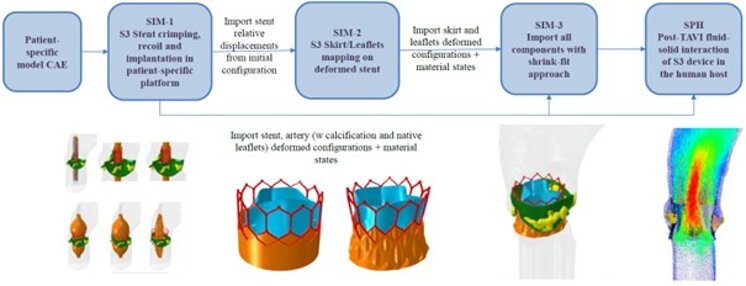
In-Silico Virtual Clinical Trial Platform
This platform was used within the SimInSitu project to conduct an In-Silico Clinical Trial (ISCT) as a Refinement Study to assess the patient risk associated with the treatment with a investigational Pulmonary Valve device. Ten paediatric patient models were included into this study. Primary and secondary endpoints were defined. The trial was comprised of three principal parts: the virtual device implantation under surgical / clinical expert guidance; the generation of in-silico data by means of patient-specific FSI analyses; and the clinical assessment of these data.
Obtained in-silico data indicate that five of the patients do not reach the primary endpoint, due to critical under-sizing with the available device sizes. Consequently the inclusion criteria of the clinical trial should be revised to ensure a higher success rate. The credibility of the used in-silico model was established through a dedicated development and hierarchy-based VVUQ strategy.
The models will be provided to the public with controlled access due to the confidential nature of the device model data. To request access, please use the provided template and describe the motivation and provide contact data and a study outline (SOW). The signed pdf-document shall be send to the SimInSitu Consortium Coordinator via the following address:
The consortium will evaluate and respond to the request within a reasonable timeframe.